Research Background: Ammonia (NH3) is a carbon-free energy carrier and a promising energy storage medium. It boasts a high gravimetric hydrogen density of 17.6%, a high energy density of 4.32 kWh L-1, and a moderate liquefaction point. Additionally, NH3 is the second most produced chemical globally, driven by its extensive use in agriculture and industrial manufacturing. Every year, more than 200 million tons of NH3 are produced from N2 and H2 using the conventional Haber-Bosch process, which requires high temperatures (400-550 °C) and pressures (150-350 atm). However, this process not only consumes a substantial amount of energy (accounting for 2% of global energy output), but also contributes to large-scale CO2 emissions due to the use of natural gas reforming to generate the required H2. To produce NH3 sustainably, researchers have proposed the electrocatalytic N2 reduction reaction (NRR). This method aims to facilitate NH3 production from N2 and H2O at ambient pressure and room temperature. However, the N2 molecule poses challenges due to its extremely high dissociation energy of 941 kJ mol-1 and the poor solubility of N2 in aqueous electrolytes. These factors result in a low NH3 production rate and Faradaic efficiency (FE). To promote ammonia synthesis in a sustainable way, the electrocatalytic nitrate reduction reaction (NITRR, NO3− + 6H2O + 8e−→ NH3 + 9OH−) offers an alternative and attractive route. This approach has several notable advantages. First, the hydrodeoxygenation of NO3− requires a reduced energy barrier due to the much lower dissociation energy (204 kJ mol-1) of the N-O bond under mild conditions. Second, the higher solubility of nitrate in aqueous electrolytes solves the mass transfer issue. Third, nitrate sources are widespread in nature, primarily attributed to human activities such as industrial emissions, vehicle exhaust, and municipal wastewater. Forth, the extensive and abundant nitrate reserves from niter ore (mainly in Chile and China's Turpan Basin) further supports the potential implementation of large-scale ammonia production through the electricity-driven NITRR. Considering these advantages, the electrochemical NITRR represents a promising green, sustainable and reliable approach with significant atomic economy, enabling the conversion of waste into valuable resources and the reduces the carbon footprint for fertilizer manufacturing. The electrocatalytic nitrate reduction reaction (NITRR) holds great promise for producing valuable ammonia (NH3). However, the lack of efficient electrocatalysts has impeded the achievement of highly selective NH3 synthesis from the NITRR.
Research Findings: In this study, we report the design and synthesis of two polynuclear Co-cluster based coordination polymers,{[Co2(TCPPDA)(H2O)5]·(H2O)9(DMF)} and {Co1.5(TCPPDA)[(CH3)2NH2]· (H2O)6(DMF)2} (namely NJUZ-2 and NJUZ-3), which possess distinct coordination motifs with well-defined porosity, high-density catalytic sites, accessible mass transfer channels, and nano-confined chemical environments. Benefited from their intriguing multi-core metal-organic coordination framework structures, NJUZ-2 and NJUZ-3 exhibit remarkable catalytic activities for the NITRR. At a potential of -0.8 V (vs. RHE) in an H-type cell, they achieve the optimal Faradaic efficiencies of approximately 98.5% and high long-term durability for selective NH3 production. Furthermore, the electrocatalytic performance is well maintained even under strongly acidic conditions. When operated under an industrially relevant current density of 469.9 mA cm-2 in a flow cell, a high NH3 yield rate of up to 3370.6 mmol h-1 g-1cat. at -0.5 V (vs. RHE) was achieved, which is 20.1-fold higher than that obtained in H-type cell under same conditions. Extensive experimental analyses, in combination with theoretical computations, reveal that the great enhancement of the NITRR activity is attributed to the preferential adsorption of NO3− and the reduction in energy input required for the hydrogenation of *NO3 and *NO2 intermediates.

Figure 1.Structural assembly. Schematic structure assembly processes and crystal structures of (upper) NJUZ-2and (bottom)NJUZ-3 CPs using organic-inorganic hybrid building blocks.

Figure 2. Material characterizations. (a, b) Experimental and simulated PXRD patterns of (a) NJUZ-2 and (b) NJUZ-3, respectively. The insets are the corresponding three-dimensional crystal structures. (c) High-resolution TEM image of NJUZ-2. (d, e) XPS spectra at Co 2p level for (d) NJUZ-2 and (e) NJUZ-3 samples, respectively. (f) Co K-edge XANES and (g) EXAFS spectra of NJUZ-2 and NJUZ-3 along with other reference samples. Note the intensity of Co foil is multiplied by a coefficient of 0.2 in EXAFS spectra for the readers’ convenience.(h)Co K-edge wavelet transformation of NJUZ-2 and NJUZ-3 samples along with other reference samples.

Figure 3. Electrocatalytic activitiesof NJUZ-2 and NJUZ-3 for the NITRR. (a) LSV curves tested in 0.1 M Na2SO4 solution with and without 0.1 M KNO3. (b, c)FE values (left Y axes) and corresponding NH3 yield rates (right Y axes) of (b) NJUZ-2 and (c) NJUZ-3 tested at different applied potentials for the NITRR. (d) LSV curves tested in 0.5 M KNO3 and 0.5 M Na2SO4 aqueous electrolyte. (e, f) FE values (left Y axes) and corresponding NH3 yield rates (right Y axes) of (e) NJUZ-2 and (f) NJUZ-3 tested at different applied potentials for the NITRR. (g) FE values and total NH3 yields for NJUZ-2 electrocatalyst during long-term NITRR stability measurement for 70 h. (h) 1H-NMR spectra of the products after the NITRR test of NJUZ-2 using K14NO3 and 15N isotope labeled Na15NO3 as the feeding nitrogen sources, respectively. (i) UV-Vis absorption spectra of the products after 0.5 h NITRR electrolysis of NJUZ-2 with a strongly acidic electrolyte (0.05 M H2SO4, pH = 1) containing 0.1 M KNO3 and colored with indophenol indicator. (j) Corresponding FE values (left Y axes) and NH3 yield rates (right Y axes) under different applied potentials.

Figure 4.In situ analyses and mechanism studies. (a) Schematic illustration of the apparatus for in situ electrochemical ATR-FTIR characterizations. (b) In situelectrochemical ATR-FTIR spectra of NJUZ-2 under different potentials, respectively. (c) The charge density difference of the NO3- adsorbed on the NJUZ-2 model. The yellow and cyan corroborate charge accumulation and charge consumption, respectively. The iso-surface value is 0.004 eV Å-3. (d) PDOS of Co-3d and NO3--2p adsorbed on the NJUZ-2 model. (e) Calculated free-energy diagrams of the possible electrocatalytic NITRR pathways on the NJUZ-2 model. The insets illustrate the optimized adsorption configurations of various reaction intermediates along the N-end and O-end pathways.
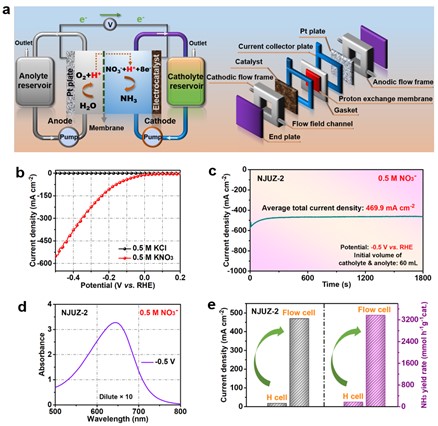
Figure 5. The NITRR test results in a flow cell. (a) Schematic illustration of the flow cell system. (b) LSV curves of NJUZ-2 electrocatalyst tested in the flow cell using aqueous electrolytes of 0.5 M KCl and 0.5 M KNO3, respectively. (c) Time-dependent industrially relevant current density curve of NJUZ-2 electrocatalyst at an applied potential of -0.5 V vs. RHE. (d) The corresponding UV-Vis absorption spectrum of the product in (c) after diluted 10 times and stained with indophenol indicator. (e) Comparisons of the as-measured current densities and NH3 yield rates measured in conventional H-type cell and flow cell under the same reaction conditions (0.5 M KNO3 electrolyte, -0.5 V vs. RHE, 0.5 h reaction time).
