Dr. Zhu-Jun Yao has published 5 books, more than 200 peer-review journal publications and 20 patents in the fields of synthetic natural product chemistry and chemical biology.
Selected recent publications:
(1) Wang, T.-Y.; Feng, D.-H.; Wang, Y.-K.; Yao, Z.-J. Quick Access to Protoberberine and Tetrahydroprotoberberine Derivatives/Analogues with One-pot Sequential Isochromenylium-mediated Annulation. J. Org. Chem. 2024, (submitted on June 19, 2024).

(2) Mai, Z.-P.; Zhang, B.; Pang, Z.-X.; Shi, J.; Xu, Z.-F.; Huang, B.-B.; Ma, S.-Y.; Jiao, R.-H.; Yao, Z.-J.; Tan, R.-X.; Ge, H.-M. A trans-AT polyketide synthase accomplishes iterative function in the biosynthesis of lankacidin-type natural products. Nat. Synth. 2024 (MS#NATSYNTH-23121423B, accepted on June 6, 2024).

(3) Nong, K.; Zhao, Y.-L.; Yi, S.; Zhang, X.; Wei, S.; Yao, Z.-J. 3-Acyl-4-pyranone as Lysine-residue Selective Bioconjugation Reagent for Peptide and Protein Modification. Bioconjugate Chem.2024, 35, 286-299. (DOI: 10.1021/acs.bioconjchem.3c00447)

(4) Wang, M.; Wang, T.; Qin, X.; Yao, Z.-J. Development of Cyclic N,O-Aminal-Embedded Bis-tetrahydroisoquinoline Analogues as Potential DNA Alkylation Agents. Org. Lett. 2024, 26, 1764-1769. (DOI: 10.1021/acs.orglett.3c04143)

(5) Wang, T.; Wang, Y.; Feng, D.; Wang, M.; Yang, X.; Yao, Z.-J. Isochromenylium/Isoquinolinium-Mediated One-Pot Annulationto Hexahydropyrazinoisoquinolines. Synthesis of Quinocarcinol. Org. Lett. 2023, 25, 8803−8808. (DOI: 10.1021/acs.orglett.3c03368; published on Dec. 6, 2023)

(6) Yi, S.; Wei, S.; Wu, Q.; Wang, H.; Yao, Z.-J. Azaphilones as Activation-Free Primary Amine-Specific Bioconjugation Reagents for Peptides, Proteins and Lipids. Angew. Chem. Int. Ed. 2022, 61(6), e202111783 (DOI: 10.1002/anie.202111783; published on Feb. 1, 2022).

(7) Tang, S.; Wu, Z.; Gao, M.; Li, G.; Yao, Z.-J. Total Synthesis of Suberitines A-D Featuring Tunable Biomimetic Late-Stage Oxidative Dearomatization and Acetalization, Chem. Eur. J. 2022, 28(24), e202200644 (DOI: 10.1002/chem.202200644).

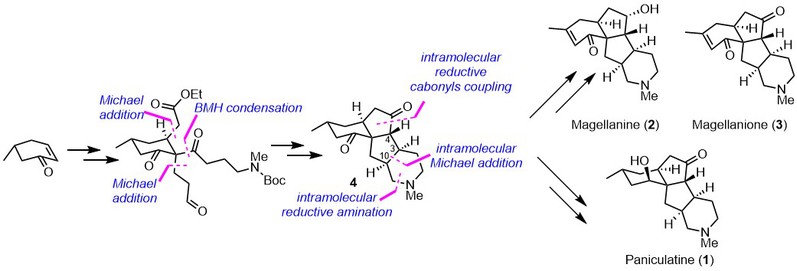
(8)Huang, B.-B.; Lei, K.; Zhong, L.-R.; Yang, X.; Yao, Z.-J. A Unified Total Synthesis of Tetracyclic Diquinane Lycopodium Alkaloids (+)-Paniculatine, (−)-Magellanine and (+)-Magellaninone. J. Org. Chem. 2022, 87, 8685-8696 (DOI: 10.1021/acs.joc.2c00871; accepted on June 3, 2022).
(9) Yu, B.-B.; Yuan, H.; Chen, Y.-C.; Zhou, D.-X.; Gan, Z.-J.; Wang, J.; Li, J.-X.; Yao, Z.-J. Annonaceous Acetogenin Mimic AA005 Inhibits the Growth of TNBC MDA-MB-468 Cells by Altering Cell Energy Metabolism. ChemBioChem 2022, e202200250. (DOI: 10.1002/cbic.202200250; accepted on May 30, 2022).

(10) Gao, M.; ‡ Yu, B.-B.;‡ Jia,C.; Yao, Z.-J. Cytotoxic analogues of marine diterpenoid plumisclerin A by shifting the lipophilic branch on the characteristic tricyclic core. Org. Biomol. Chem. 2022, 20, 4553-4558. (‡These authors contributed equally to this work. DOI: 10.1039/D2OB00539E; accepted on April 12).

(11) Huang, B.-B.;# Zhao, Y.-L.;# Lei, K.; Zhong, L.-R.; Yang, X.; Yao, Z.-J. Enantioselective Total Synthesis of (+)-Sieboldine A and Analogues Thereof. Org. Lett. 2022, 24, 7517−7521. (Accepted on October 7, 2022; DOI: 10.1021/acs.orglett.2c02737). (#These authors contributed equally to this work)

(12) Wang, M.; Yu, B.-B.; Yao, Z.-J. Simplified hybrids of two anticancer bistetrahydroisoquinoline alkaloidsecteinascidin 743 and cribrostatin 4 and inhibitory activity against proliferation of cancer cells. Org. Biomol. Chem. 2022, 20, 8438 - 8442. (DOI: 10.1039/D2OB01707E; accepted on October 11, 2022, first published on October 11, 2022)

(13) Zhong, L.-R.; Huang, B.-B.; Yang, X.-L.; Wang, S.-z.; Yao, Z.-J. Concise Unified Access to (−)-8-Deoxy-13-dehydroserratinine, (+)-Fawcettimine, (+)-Fawcettidine, and (−)-8-Deoxyserratinine Using a Direct Intramolecular Reductive Coupling. Organic Letters 2021, 23, 3578−3583.
(14) Xie, J.;† Zhu, G.;† Gao, M.;† Xi, J.; Chen, G.; Ma, X.; Yan, Y.; Wang, Z.; Xu, Z.-J.; Chen, H.-J.; Hao, H.-D.; Zhang, Y.; Yao, Z.-J.; Zhu, J. Artemisinin derivative ART1 induces ferroptosis by targeting the HSD17B4 protein essential for lipid metabolism and direct induction of lipid peroxidation. CCS Chemistry 2021, 3, 664–677 (†These authors contributed equally to this work).
(15) Qiao, J.-H.; Zhao, W.-X.; Liang, Y.; Yao, Z.-J.; Wang, S.-z. Diastereospecific Access to Tetracyclic Eight-Membered Lactams through a Dearomative Heck Reaction and an Alkylative Ring-Opening Driven by the Photoexcited Spiroindolines. Chem. Eur. J. 2021, 27, 6308–6314.
(16) Zhong, L.-R.; Yang, Y.; Huang, B.-B.; Yao, Z.-J. An eight-step total synthesis of pyrroloquinolone-type Lycopodium alkaloid via a tandem annulation approach. Chin. J. Chem. 2020, 38, 1560-1564.
(17) Shan, H.-z; Cao, Y.; Xiao, X.-h.; Liu, M.; Wu, Y.-z.; Zhu, Q.; Xu, H.-z.; Lei, H.; Yao, Z.-J.;, Wu, Y.-L. YL064 activates proteasomal-dependent degradation of c-Myc and synergistic with ABT-199 in diffuse large B cell lymphoma. Signal Transduction and Targeted Therapy 2020, 5, 116.
(18)Gao, M.; Wang, Y.-C.; Yang, K.-R.; He, W.; Yang, X.-L.; Yao, Z.-J. Enantioselective Total Synthesis of (+)-Plumisclerin A. Angew. Chem. Int. Ed. 2018, 57, 13313-13318.
(19) Zhang, L.; Wang, Y.; Yao, Z.-J.; Wang, S.-z.; Yu, Z.-X. Kinetic or Dynamic Control on a Bifurcating Potential Energy Surface? An Experimental and DFT Study of Gold-Catalyzed Ring Expansion and Spirocyclization of 2-Propargyl-β-tetrahydrocarbolines. J. Am. Chem. Soc. 2015, 137, 13290–13300.
(20) Yu, S.-Y.; Zhang, H.; Gao, Y.; Mo, L.; Wang, S.-z.; Yao, Z.-J. Asymmetric Cascade Annulation Based on Enantioselective Oxa Diels−Alder Cycloaddition of in Situ Generated Isochromenyliums by Cooperative Binary Catalysis of Pd(OAc)2 and (S)-Trip. J. Am. Chem. Soc. 2013, 135, 11402−11407.
(21) Ge, H. M.; Zhang, L.-D.; Tan, R. X.; Yao, Z.-J.Protecting Group-Free Total Synthesis of (-)-Lannotinidine B. J.Am. Chem. Soc. 2012, 134, 12323-12325.
(22) Yang, Z.-Y.; Liao, H.-Z.; Sheng, K.; Chen, Y.-F.; Yao, Z.-J. Enantioselective Total Synthesis of Marine Diterpenoid Clavulactone. Angew. Chem. Int. Ed. 2012, 51, 6484-6487.
For more details, please browse Dr. Yao's group webpage at http://hysz.nju.edu.cn/yaozj/.














