姚祝军课题组近年来致力于拓展生理药理活性天然与非天然小分子有机化合物的化学空间与生物空间,并促进二者的交汇融合,发展和运用分子探针与分子工具开展作用机制相关的化学生物学研究。取得的重要成果包括:
(1)完成了chlorofusin,chloptosin,lannotinidine B,cyclomarin,clavulactone,plumisclerin A,suberitines A-D等具有复杂结构的重要生理活性天然产物的首次全合成。
(2)通过合成设计和方法创新相互结合追求药用天然产物更高效率的获取途径,成功建立了路线简短、产率较高的著名生物碱喜树碱和10-羟基喜树碱、抗癌药物伊利替康的对映选择性全合成路线,并发展出活性更强的新颖衍生物;从经济易得的葡萄糖和氨基酸等原料出发,完成了著名抗流感药物乐感清和达菲的新合成路线;发展并应用多功能有机小分子催化方法,提供了第一条高对映选择性的石杉碱甲催化不对称合成技术路线。

Fig 1. 天然产物全合成(部分合成的化学结构)
(3)根据“结构展现功能”思想进行类天然产物结构创新,设计发明了具有细胞选择性的强抗肿瘤活性人工化合物AA005,并通过化学和生物学交叉研究手段基本阐明了AA005 在肿瘤细胞和正常细胞之间产生选择性的特殊机制;研究了中药有效成分青藤碱的后期修饰新方式,发展了一批结构独特多样的免疫调节活性、抗多发性骨髓瘤活性和乙酰胆碱酯酶抑制活性的青藤碱衍生物;剖析了阿扎菲酮与伯胺之间的交联反应机理,并成功开发了残基选择性的蛋白质赖氨酸修饰技术,用于新结构组成的抗体-药物偶联物的研发。

Fig 2. 天然产物化学生物学(部分工作)
(4)提出了全新的固载化分子反应器循环运作机制,突破了含有N-Me化或α, α-双取代等大位阻氨基酸的大位阻多肽的高效率固相合成,并与商品化多肽自动合成平台(仪)实现无缝对接,籍此大幅度扩展那些“更像药物”的高位阻合成肽的范围和类型,促进大规模的、以前不可行的肽结构优化研究。
近期发表的部分工作:
(1) Han, B.; Li, Z.-M.; Zhao, X.-Y.; Liang, K.; Mao, Y.-Q.; Zhang, S.-L.; Huang, L.-Y.; Kong, C.-Y.; Peng, X.; Chen, H.-L.; Huang, J.-T.; Yao, J.-Q.; Cai, P.-R.; Zhang, Z.-Y.; Zhang, X.-M.; Yao, Z.-J.; Chen, G.-Q.; Wang, L.-S. Annonaceous acetogenins mimic AA005 targets mitochondrial trifunctional enzyme alpha subunit to treat obesity in male mice. Nat. Commun. 2024, 15, 9100. (accepted on Sept. 27, 2024; DOI: 10.1038/s41467-024-53118-3)

(2) Wang, T.-Y.; Feng, D.-H.; Wang, Y.-K.; Yao, Z.-J. Quick Access to Protoberberine and Tetrahydroprotoberberine Derivatives/Analogues with One-pot Sequential Isochromenylium-mediated Annulation. J. Org. Chem. 2024, 89, 12853–12857 (published on August 27, 2024). (DOI: 10.1021/acs.joc.4c01540)

(3) Mai, Z.-P.; Zhang, B.; Pang, Z.-X.; Shi, J.; Xu, Z.-F.; Huang, B.-B.; Ma, S.-Y.; Jiao, R.-H.; Yao, Z.-J.; Tan, R.-X.; Ge, H.-M. A trans-AT polyketide synthase accomplishes iterative function in the biosynthesis of lankacidin-type natural products. Nat. Synth. 2024, 3, 1255-1265. (accepted on June 6, 2024; published on July 09, 2024; DOI: 10.1038/s44160-024-00599-1).

(4) Nong, K.; Zhao, Y.-L.; Yi, S.; Zhang, X.; Wei, S.; Yao, Z.-J. 3-Acyl-4-pyranone as Lysine-residue Selective Bioconjugation Reagent for Peptide and Protein Modification. Bioconjugate Chem.2024, 35, 286-299. (DOI: 10.1021/acs.bioconjchem.3c00447)

(5) Wang, M.; Wang, T.; Qin, X.; Yao, Z.-J. Development of Cyclic N,O-Aminal-Embedded Bis-tetrahydroisoquinoline Analogues as Potential DNA Alkylation Agents. Org. Lett. 2024, 26, 1764-1769. (DOI: 10.1021/acs.orglett.3c04143)

(6) Wang, T.; Wang, Y.; Feng, D.; Wang, M.; Yang, X.; Yao, Z.-J. Isochromenylium/Isoquinolinium-Mediated One-Pot Annulationto Hexahydropyrazinoisoquinolines. Synthesis of Quinocarcinol. Org. Lett. 2023, 25, 8803−8808. (DOI: 10.1021/acs.orglett.3c03368; published on Dec. 6, 2023)

(7) Yi, S.; Wei, S.; Wu, Q.; Wang, H.; Yao, Z.-J. Azaphilones as Activation-Free Primary Amine-Specific Bioconjugation Reagents for Peptides, Proteins and Lipids. Angew. Chem. Int. Ed. 2022, 61(6), e202111783 (DOI: 10.1002/anie.202111783; published on Feb. 1, 2022).

(8) Tang, S.; Wu, Z.; Gao, M.; Li, G.; Yao, Z.-J. Total Synthesis of Suberitines A-D Featuring Tunable Biomimetic Late-Stage Oxidative Dearomatization and Acetalization, Chem. Eur. J. 2022, 28(24), e202200644 (DOI: 10.1002/chem.202200644).

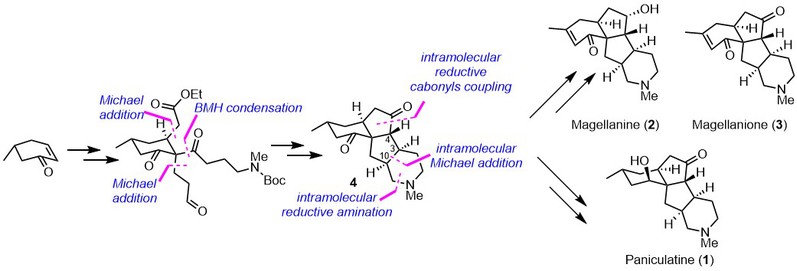
(9) Huang, B.-B.; Lei, K.; Zhong, L.-R.; Yang, X.; Yao, Z.-J. A Unified Total Synthesis of Tetracyclic Diquinane Lycopodium Alkaloids (+)-Paniculatine, (−)-Magellanine and (+)-Magellaninone. J. Org. Chem. 2022, 87, 8685-8696 (DOI: 10.1021/acs.joc.2c00871; accepted on June 3, 2022).
(10) Yu, B.-B.; Yuan, H.; Chen, Y.-C.; Zhou, D.-X.; Gan, Z.-J.; Wang, J.; Li, J.-X.; Yao, Z.-J. Annonaceous Acetogenin Mimic AA005 Inhibits the Growth of TNBC MDA-MB-468 Cells by Altering Cell Energy Metabolism. ChemBioChem 2022, e202200250. (DOI: 10.1002/cbic.202200250; accepted on May 30, 2022).

(11) Gao, M.; ‡ Yu, B.-B.;‡ Jia,C.; Yao, Z.-J. Cytotoxic analogues of marine diterpenoid plumisclerin A by shifting the lipophilic branch on the characteristic tricyclic core. Org. Biomol. Chem. 2022, 20, 4553-4558. (‡These authors contributed equally to this work. DOI: 10.1039/D2OB00539E; accepted on April 12).

(12) Huang, B.-B.;# Zhao, Y.-L.;# Lei, K.; Zhong, L.-R.; Yang, X.; Yao, Z.-J. Enantioselective Total Synthesis of (+)-Sieboldine A and Analogues Thereof. Org. Lett. 2022, 24, 7517−7521. (Accepted on October 7, 2022; DOI: 10.1021/acs.orglett.2c02737). (#These authors contributed equally to this work)

(13) Wang, M.; Yu, B.-B.; Yao, Z.-J. Simplified hybrids of two anticancer bistetrahydroisoquinoline alkaloidsecteinascidin 743 and cribrostatin 4 and inhibitory activity against proliferation of cancer cells. Org. Biomol. Chem. 2022, 20, 8438 - 8442. (DOI: 10.1039/D2OB01707E; accepted on October 11, 2022, first published on October 11, 2022)

(14) Zhong, L.-R.; Huang, B.-B.; Yang, X.-L.; Wang, S.-z.; Yao, Z.-J. Concise Unified Access to (−)-8-Deoxy-13-dehydroserratinine, (+)-Fawcettimine, (+)-Fawcettidine, and (−)-8-Deoxyserratinine Using a Direct Intramolecular Reductive Coupling. Organic Letters 2021, 23, 3578−3583.
(15) Xie, J.;† Zhu, G.;† Gao, M.;† Xi, J.; Chen, G.; Ma, X.; Yan, Y.; Wang, Z.; Xu, Z.-J.; Chen, H.-J.; Hao, H.-D.; Zhang, Y.; Yao, Z.-J.; Zhu, J. Artemisinin derivative ART1 induces ferroptosis by targeting the HSD17B4 protein essential for lipid metabolism and direct induction of lipid peroxidation. CCS Chemistry 2021, 3, 664–677 (†These authors contributed equally to this work).
(16) Qiao, J.-H.; Zhao, W.-X.; Liang, Y.; Yao, Z.-J.; Wang, S.-z. Diastereospecific Access to Tetracyclic Eight-Membered Lactams through a Dearomative Heck Reaction and an Alkylative Ring-Opening Driven by the Photoexcited Spiroindolines. Chem. Eur. J. 2021, 27, 6308–6314.
(17) Zhong, L.-R.; Yang, Y.; Huang, B.-B.; Yao, Z.-J. An eight-step total synthesis of pyrroloquinolone-type Lycopodium alkaloid via a tandem annulation approach. Chin. J. Chem. 2020, 38, 1560-1564.
(18) Shan, H.-z; Cao, Y.; Xiao, X.-h.; Liu, M.; Wu, Y.-z.; Zhu, Q.; Xu, H.-z.; Lei, H.; Yao, Z.-J.;, Wu, Y.-L. YL064 activates proteasomal-dependent degradation of c-Myc and synergistic with ABT-199 in diffuse large B cell lymphoma. Signal Transduction and Targeted Therapy 2020, 5, 116.
(19)Gao, M.; Wang, Y.-C.; Yang, K.-R.; He, W.; Yang, X.-L.; Yao, Z.-J. Enantioselective Total Synthesis of (+)-Plumisclerin A. Angew. Chem. Int. Ed. 2018, 57, 13313-13318.
(20) Zhang, L.; Wang, Y.; Yao, Z.-J.; Wang, S.-z.; Yu, Z.-X. Kinetic or Dynamic Control on a Bifurcating Potential Energy Surface? An Experimental and DFT Study of Gold-Catalyzed Ring Expansion and Spirocyclization of 2-Propargyl-β-tetrahydrocarbolines. J. Am. Chem. Soc. 2015, 137, 13290–13300.
(21) Yu, S.-Y.; Zhang, H.; Gao, Y.; Mo, L.; Wang, S.-z.; Yao, Z.-J. Asymmetric Cascade Annulation Based on Enantioselective Oxa Diels−Alder Cycloaddition of in Situ Generated Isochromenyliums by Cooperative Binary Catalysis of Pd(OAc)2 and (S)-Trip. J. Am. Chem. Soc. 2013, 135, 11402−11407.
(22) Ge, H. M.; Zhang, L.-D.; Tan, R. X.; Yao, Z.-J.Protecting Group-Free Total Synthesis of (-)-Lannotinidine B. J.Am. Chem. Soc. 2012, 134, 12323-12325.
(23) Yang, Z.-Y.; Liao, H.-Z.; Sheng, K.; Chen, Y.-F.; Yao, Z.-J. Enantioselective Total Synthesis of Marine Diterpenoid Clavulactone. Angew. Chem. Int. Ed. 2012, 51, 6484-6487.
更多信息,请浏览姚祝军教授课题组主页:http://hysz.nju.edu.cn/yaozj/.

















